TMCnet News
Perosphere Technologies Announces CE-IVD Marking of the Perosphere Technologies PoC Coagulometer System for DOAC and Heparin TestingDANBURY, Conn., May 7, 2021 /PRNewswire/ -- Perosphere Technologies Inc. today announced CE (Conformité Européene)-IVD Marking of its Point-of-Care (PoC) Coagulometer System. The CE Mark confirms that the Company's PoC Coagulometer System meets the safety requirements of the European In-Vitro Diagnostic Devices Directive (98/79/EC) and allows Perosphere Technologies to begin commercialization of its products throughout the European Union and other CE Mark geographies. 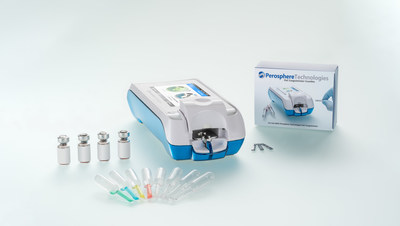 The Perosphere Technologies PoC Coagulometer is a hand-held device, equipped with a color touch screen, barcode reader, and an eight hour run-time battery for use at the patient's bedside. The PoC Coagulometer technology measures the intrinsic clotting time of fresh whole blood, providing an unprecedented look at blood coagulability, addressing several unmet medical needs. In particular, the PoC Coagulometer is the first point of care device to enter the market specifically designed for direct oral anticoagulant (DOAC, e.g., rivaroxaban, apixaban, and edoxaban) and low molecular weight heparin (LWMH) coagulation testing in both emergency and outpatient settings. Performing a measurement is simple and easy, requiring only 14 microliters of fresh venous whole blood to load into a single-use cuvette. The result is a whole blood clotting time (WBCT, reported as a clotting time in seconds) that is available in 4-6 minutes, and is used to determine if a patient is appropriately anticoagulated. Worldwid, approximately 1 million DOAC patients annually present to the emergency department with severe bleeding (e.g. gastrointestinal bleeding or intracranial hemorrhage) or requiring emergency surgery. Globally, the "high risk" DOAC and LWWH patient populations are approximately 5 million and 3 million patients annually, respectively, representing those individuals with conditions such as renal or hepatic insufficiency, low BMI, or a history of major bleeding or thrombosis for whom measuring WBCT at intervals could improve quality of care. "We are excited to offer healthcare providers in the EU and other international markets a first-in-class solution for DOAC and low molecular weight heparin coagulation testing at the point of care," said Dr. Sasha Bakhru, CEO of Perosphere Technologies. "We believe our PoC Coagulometer system will change the paradigm for managing DOAC and heparin therapy in emergency settings and beyond." Perosphere Technologies plans to launch the PoC Coagulometer system in select EU countries later this year, with an expanded launch planned for early Q1 2022. If you are interested in learning more about the PoC Coagulometer, please contact us at [email protected] or by phone at +1 (475) 218-4600. About Perosphere Technologies Perosphere Technologies is a private medical technologies company, focused on the development and commercialization of a novel Point-of-Care (PoC) coagulometer technology platform. For further information, contact us at [email protected] or +1 (475) 218-4600. Contacts Perosphere Technologies Inc. or Daryl Mootoo  Photo - https://mma.prnewswire.com/media/1505266/Perosphere_Technologies_B_Radius8_Smoothing4__noBezelSmooth.jpg 
|
